Enquire Form
Home >> TAGLUS TUFF
-
.png)
Taglus Tuff
Revolutionary engineered plastic for Retainers
Uniaxially oriented amorphous material with polymer chain locked together in a non specific lattice structure Our addition of special grade glycol to PET removes the hazing effect seen during heating and also prevents an undesirable crystallization.
Additionally, the inclusion of glycol in this composition transforms the inner walls of aligner/ retainer into a more comfortable material to the patient. So TAGLUS Tuff is unique engineering combination of elasticity with rigidity and clarity – a perfect balance
-

- Ultra Thin: Taglus Tuff is the thinnest retainer of 0.8mm thickness . Its minimum thickness makes it ultra comfortable to wear.
- Ideal Mechanical Properties: The Significant high break strength of 59 MPa keeps the sheet from breaking. The high level of ductility is provided by 7% Yield elongation.
- Predictable Retaining Force : It provides sufficient yield strength (41 MPa) to retain the teeth’ position.
- Dual Protective masking : Ultra-thin, peel-away masking on both sides ensures that it remains scratch-proof, moisture resistant, and promotes shelf life.
characteristics
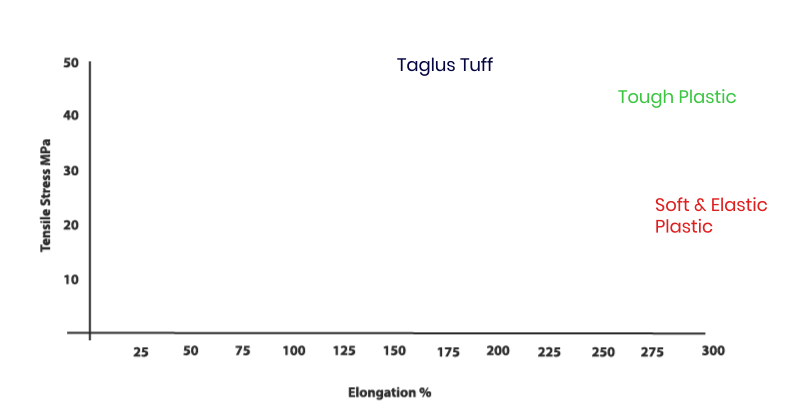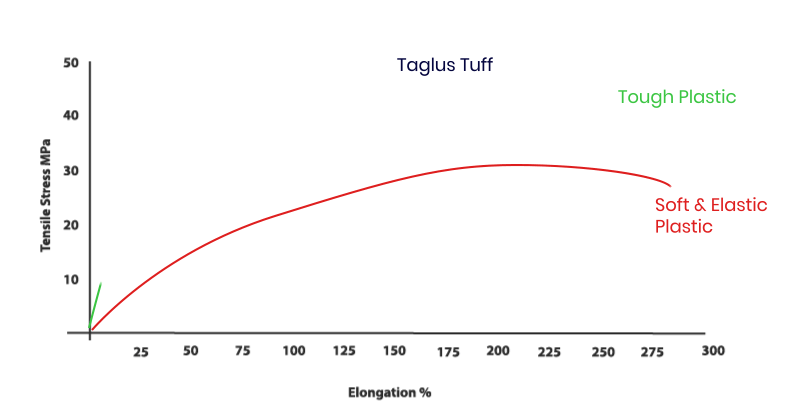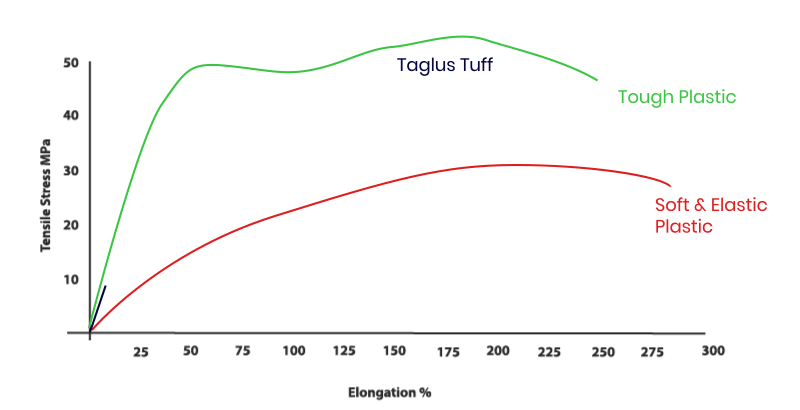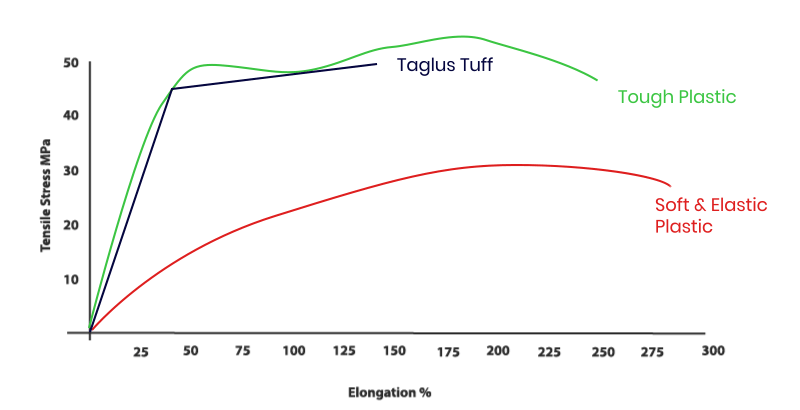
Our addition of special grade glycol to PET removes the hazing effect seen during heating and also prevents an undesirable crystallization. Additionally, the inclusion of glycol in this composition transforms the inner walls of aligner/ retainer into a more comfortable material to the patient. So TAGLUS Tuff is unique engineering combination of elasticity with rigidity and clarity – a perfect balance.
The properties of TAGLUS Tuff sheets when investigated using standardized test methods, e.g. tensile stress as per ASTM D 638: 2014 by briefly applying load in one direction the approximate results and values observed during such test, demonstrate that TAGLUS Tuff sheets in a unique balance of strength and toughness. The test was performed by an NABL accredited Laboratory complying with ISO/OEC 17025 Laboratory Management System.

Tensile Modulus, or Youngs Modulus, widely known as the tendency of an object to deform along an axis when opposing forces are applied along that axis; it is defined as the ratio of tensile stress to tensile strain. TAGLUS Tuff having a very high tensile modulus of approximately upto 1872MPa, tested as per ASTM D638:2014 makes it the best comfortable retainer
The photographs of TAGLUS Tuff when compared with other PU based aligner sheets shows a noticeable difference in the clarity of the object placed at a distance from the sheet. TAGLUS Tuff has highest clarity in its class.
FAQ
have any question
Is it clinically tested? How safe is it to use in the mouth?
Taglus Tuff™ sheet have passed biocompatibility testing namely Skin Sensitization, in vitro Cytotoxicity and Skin Irritation test as a regulatory requirement for demonstrating the preclinical safety of medical devices, this is evaluated in accordance with the standard guideline, published by the US FDA” Use of International Standard ISO 10993-1, “Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process” which can be assessed at https://www.fda.gov/media/85865/download, issued on September 4th 2020 and originally published on 16th June 2016.
CERTIFICATIONS
- CE
- TGA
- ANVISA
What material is it made of?
The uni-axially oriented amorphous structure has polymer chains locked together in a specific lattice structure. This leads to the formation of Taglus tuff, which is a ground-breaking material superior to regular PETG materials Taglus Tuff is *Patent Pending* in India
How strong is it? Does it crack under pressure or after biting?
Taglus Tuff Sheets exhibit a tensile strength of 1872MPa making them crack-free even after prolonged use in ideal cases.
What does the peel away mask do?
The Taglus tuff material sheet is covered on both sides with an ultra-thin layer of protective covering. This peel-away masking does not get removed during thermoforming procedure. It shields the retainer from getting exposed to dust and other substances that can affect its surface. Thus the optical clarity is preserved forming an ultra-clear final product.
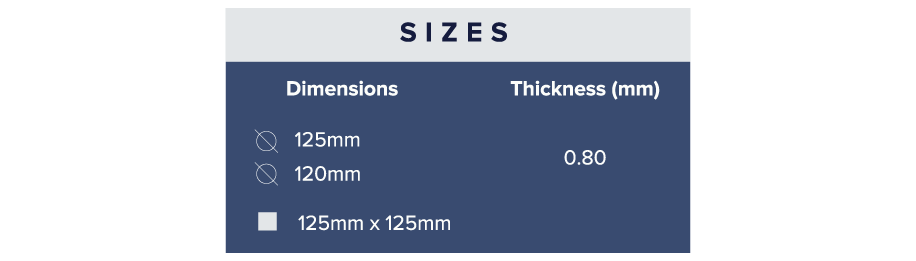
What are the various commercially available dimensions and thicknesses of these sheets?
Available in a wide variety of thickness and shapes refer Table. Customized “Run-to-Size” dimensions are also available.
What are working codes for Taglus tuff ?
Taglus Tuff can be used with several commercially available thermoforming units. For working codes, kindly refer ( brochure link)
Is it easily available all over?
Yes, Taglus Premium sheets are have an extensive global distribution. Kindly contact us +91-9930905047
What’s the shelf life?
The shelf life is 03 years subject to non-opening of vacuum-packed packaging.










